Measure Static Charge
- Published: April 01, 2010, By By Dr. Kelly Robinson Contributing Editor
Charges, electrons, and ions cause static problems. When chemically different materials touch and separate, one will have positive charge, and the other will have negative charge. This separation of charge causes dust attraction, sheet sticking, media jams, and static sparks.
Measuring static is important so we can set specification, determine if static levels are acceptable, and know when to take action when static is too high. There are at least three instruments we can use to measuring static: electrostatic fieldmeters, Coulomb meters used with Faraday cups, and non-contacting electrostatic voltmeters.
The only meter that actually measures charge is the Coulomb meter. And, since charges are at the root of static problems, the Coulomb meter should be the best one to use…right?
Let's take a look at how a Coulomb meter works and how we can use it to measure static charge. We will find that while Coulomb meters are useful, electrostatic fieldmeters and non-contacting electrostatic voltmeters usually are better choices when solving static problems in converting operations.
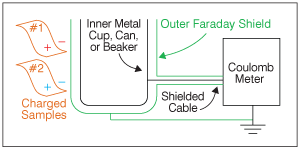
The Coulomb meter is named in honor of the French physicist Charles-Augustin de Coulomb, who developed the law for the force between electrical charges. Let's use a Coulomb meter to measure the charge on two film samples. In Figure 1, the static charge on each of the film samples induces an equal and opposite “image charge” in the grounded metal shield shown in green. For clarity, the charges associated with sample #1 are red and the charges for sample #2 are blue.
It is very important to see that no charge is induced on the inner metal cup. This combination of an outer shield surrounding an inner electrode is called a Faraday cup in honor of the English chemist and physicist Michael Faraday, who first demonstrated that the outer electrode shields the inner cup from external electric fields.
In Figure 2, we have placed film sample #1 inside the Faraday cup. Now, the charge on sample #1 induces an equal and opposite image charge on the inner electrode shown in black. This image charge moved from the outer (green) shield through the Coulomb meter to the inner (black) cup. The Coulomb meter measures this image charge.
Since we know that it is equal and opposite to the charge on the sample, the meter automatically switches the sign and displays the result, usually in units of nano-Coulombs (10-9 Coulombs).
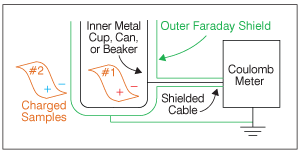
Next, we place sample #2 inside the Faraday cup, and our Coulomb meter displays the sum of the charges on Sample #1 and Sample #2. Coulomb meters have a “zero” button to reset the measurement, so we could measure just the charge on sample #2.
Now that we understand how a Coulomb meter works, it is easy to see why this instrument is awkward when measuring the charge on a continuous film. To measure the charge, our film sample must fit inside the Faraday cup. While I have often cut film samples to measure charge, I need to ask the operators to stop the converting operation to cut my samples. I often wonder if stopping the process changed the film charge so that my cut samples are not a very good measure of the charge on the film when the process is running. And, I need to handle the samples carefully so the charge that I measure is “process” charge rather than “handling” charge.
While Coulomb meters are useful and have their place solving static problems, measuring charge on moving film in a converting operation requires a non-contacting sensor such as an electrostatic fieldmeter or an electrostatic voltmeter.
Static control expert Dr. Kelly Robinson, president of Electrostatic Answers, has 27+ years of experience in problem-solving and consulting. Contact him at 585-425-8158; kelly.robinson@electrostaticanswers.com; www.electrostaticanswers.com.













