The Relationship of Surface Characteristics and Successful Corona Treatment
- Published: January 17, 2022
By: Alyxandria Klein, Contributing Writer
The way a material responds to treatment is directly related to the material’s molecular structure. Every material’s structure is different and should be treated so when deciding on a watt density level (power level and exposure time to corona discharge).
The crystallinity of polymers effects the rate that their surface energies increase. As the crystallinity of the polymer increases (from lower to higher, PE, PEP, PP), longer exposure time is needed to increase the surface energy.
Corona Treatment Definition
Corona treatment is a scientifically proven surface modification process that has been practiced in various forms for over 70 years. The object of surface treatment as a corona process is to render a typical plastic film or surface receptive to inks, glues and coatings. The process is most widely known for its ability to improve the surface bonding characteristics of various materials.
Understanding Surface Energy
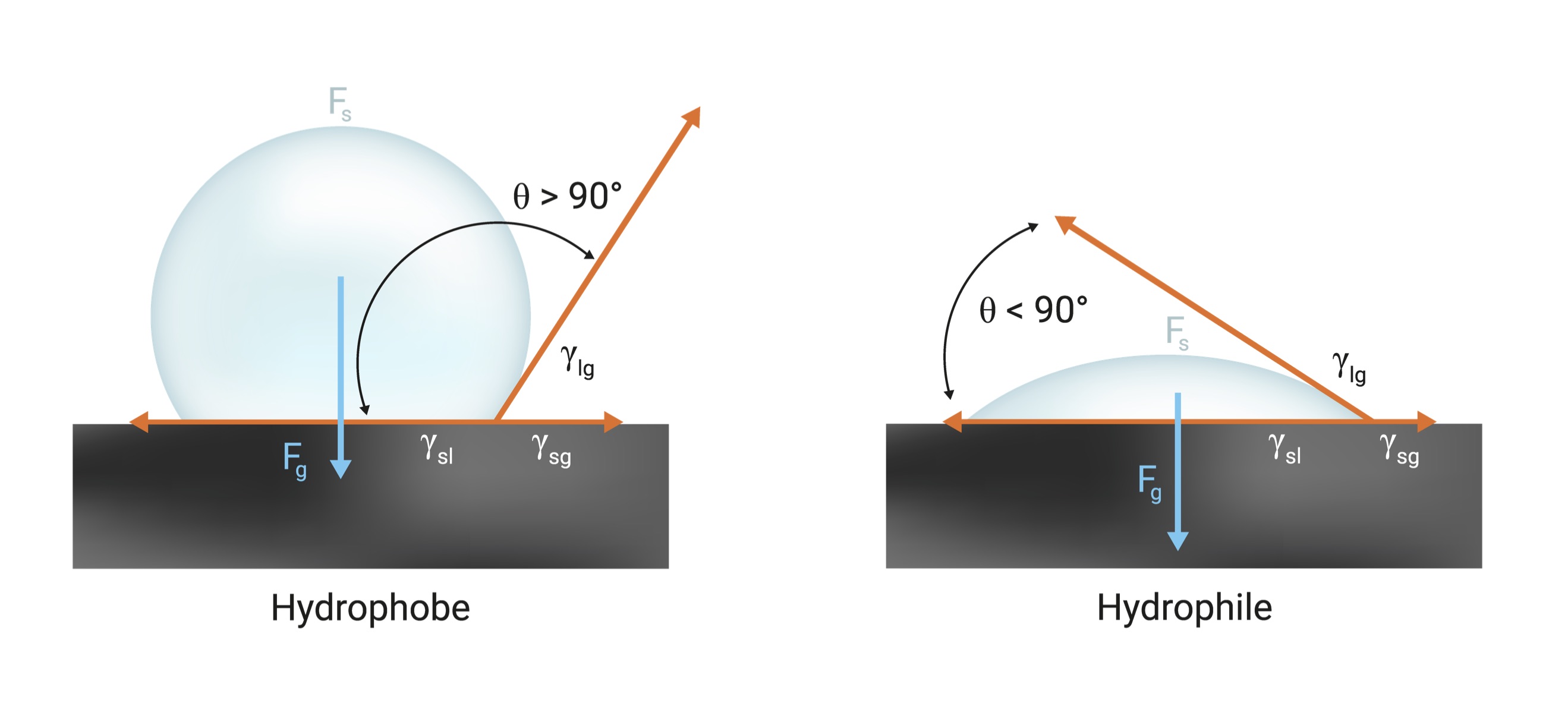
Corona treatment introduces polar functional groups such as hydroxyl, carbonyl and carboxylic groups onto the film surfaces. The presence of these functional groups on the film surface raises the film surface-free energy. Understanding a material’s surface energy is vital to a successful process, especially when coating, laminating or printing. The surface energy (or surface tension) of a liquid is the amount of excess energy at the surface of the liquid. Surface tension exists because molecules in the bulk liquid are in a lower energy state at the surface. When a liquid droplet is placed on a solid surface, what happens depends on the relative surface energy of the liquid compared to the surface energy of the solid. If the liquid has a higher surface energy than the attractive forces between the liquid and the solid surface, the liquid droplet will prefer to maintain its spherical form. This type of droplet is called a hydrophobe. On the other hand, when the surface energy of the liquid is lower than the surface of the solid, the liquid will wet out on the solid surface. This phenomenon is referred to as a hydrophile. For an adhesive to wet a solid surface, the adhesive should have a lower surface tension than the critical surface tension of the solid. This is the reason for surface treatment of plastics, which increases their surface energy and polarity.
Determining Target Surface Energies
The target surface energy of a material depends on the process and coating. Printing processes will require different surface energies than laminating or coating processes. The type of printing process (Flexo and Gavure, Litho, Offset/Letterpress, Screen and Pad) should be considered, as well as coating type (water, solvent or UV). Waterborne adhesives universally require higher surface energy levels than solvent-based systems. Table 1 provides some recommended values based on process and coating type used.
Means of Measurement Dyne Testing
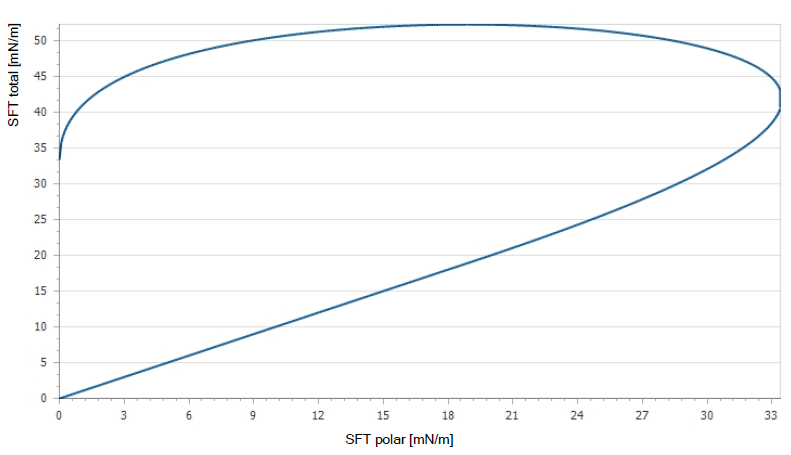
Dyne testing is common for estimating the treatment level of plastic surfaces in production environments. It involves using solutions made from a mixture of two chemicals that produce liquids (dyne) with surface tensions in the typical range of 30-70 dynes/cm. The test consists of applying various dyne liquids to the treated surface and observing the spreading of the drops over two seconds. This method allows for quick and easy estimates of treatment levels, which makes it useful and popular in production environments. A more quantitative approach is the measurement of contact angle, which decreases with an increase in treatment level. A perfect wetting liquid forms a contact angle of zero on the solid surface.
Contact Angle Measurement
Wettability may also be determined by measuring the contact angle between the surface and the drop of a liquid such as distilled water. A small contact angle indicates that the liquid is wetting the polymer effectively, while large contact angles show that the wetting is poor. This company utilizes a surface analyzer to measure surfaces and collect data on all samples. The surface analyzer places a droplet of water onto the surface along with a droplet of diiodomethane (polar element required to calculate precise surface free energy). The analyzer measures and records the contact angle of the droplets using a microscope and calculates the surface free energy. A wettability report is then generated including a wetting envelop.
Maintaining Material Integrity
Corona treatment is not a mechanical bonding process. A corona treater should be designed to allow the least physical roughening to the material as possible. A simple way to tell if the corona treater has maintained integrity in an R&D setting is to run your hand along the treated area of the film. If you can physically feel the film is rougher in the area that was corona treated, this is a sign of unnecessary roughening. The corona treater changes the molecular properties to increase surface energy to a compatible level of the application. When performed correctly the process does not alter the physical appearance or texture.
Corona treatment is a great process for improving the bonding characteristics of materials when the science and physics of corona treatment are followed correctly. This process allows companies to expand their horizons and offer a more profitable, high-quality product. There are many variables that go into deciding a target treatment level (surface energy), and all should be considered to get the most out of corona treatment and its surface modification capabilities.
References
Adhesion and Adhesives: Science and Technology; Anthony J. Kinloch, New York: Chapman and Hall (1987)
Adhesives Technology Handbook: 3rd Edition; Sina Ebnesajjad & Arthur H. Landrock: Elsevier (2014)
Alyxandria Klein is the Marketing and Sales Director, QC Electronics, Inc. To assist in her role, Klein utilizes sophisticated surface analyzation equipment to study the molecular structure of materials pre and post corona treatment. She can be reached at sales@qcelectronics.com.